

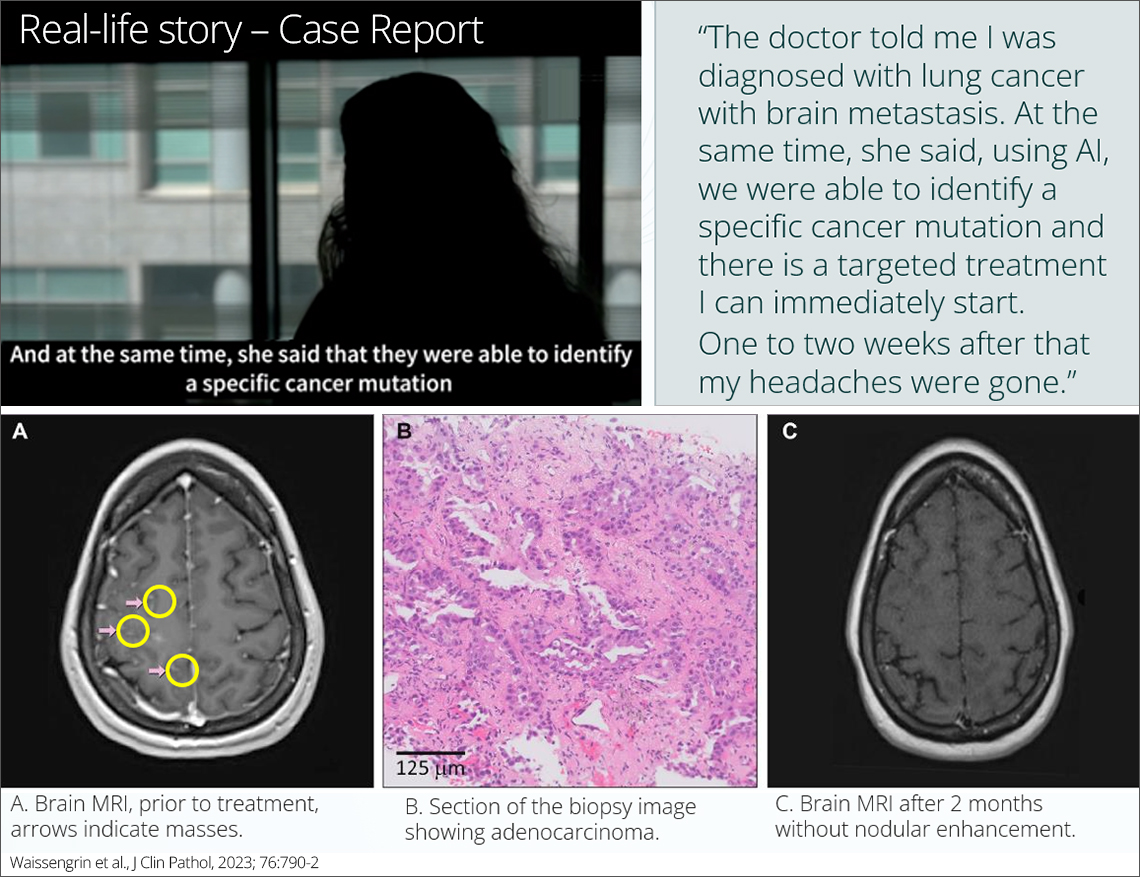
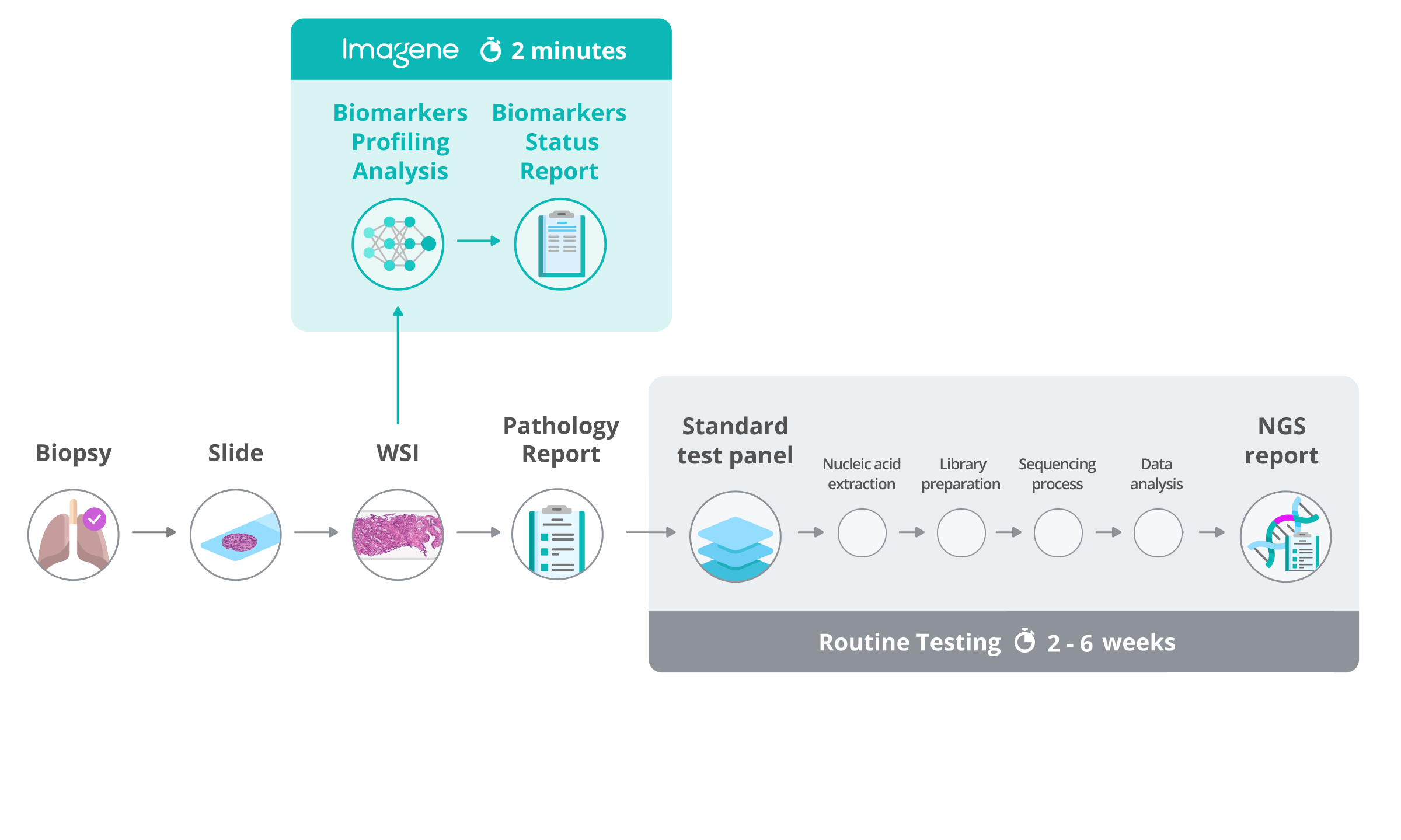
LungOI, the first AI-powered multi-gene biomarker profiling test to identify actionable alterations in NSCLC patients.
A Laboratory Developed Test (LDT) providing next day results, clinically available through a single CLIA-certified laboratory in Phoenix AZ.
No additional tissue is required, our lab can receive a glass slide or a digital H&E scan.
Rapid profile of NSCLC biomarkers from the biopsy image alone


Imagene's lab is located at 2415 East Camelback Road, Suite 700, Phoenix, AZ 85016.
Hours of Operation: Monday through Friday
9:00 a.m. to 5:00 p.m. (MST, GMT-7).
AI-based Non-Small Cell Lung Cancer (NSCLC) biomarker profiling test using H&E whole slide images (WSIs).
The Lab can receive a glass slide or a digital H&E scan. The lab will provide a status biomarkers profiling report to authorized clinicians.
Imagene's lab is located at 2415 East Camelback Road, Suite 700, Phoenix, AZ 85016.
Hours of Operation: Monday through Friday
9:00 a.m. to 5:00 p.m. (MST, GMT-7).
AI-based Non-Small Cell Lung Cancer (NSCLC) biomarker profiling test using H&E whole slide images (WSIs).
The Lab can receive a glass slide or a digital H&E scan. The lab will provide a status biomarkers profiling report to authorized clinicians.
*Imagene AI will conducts the LungOI test at 2415 East Camelback Road, Suite 700, Phoenix, AZ 85016. The lab is CLIA certified to conduct clinical laboratory testing. This test has not been cleared or approved by the U.S. Food and Drug Administration. Test ordering is limited to authorized clinicians only.
| Cookie | Duration | Description |
|---|---|---|
| __hssrc | session | This cookie is set by Hubspot whenever it changes the session cookie. The __hssrc cookie set to 1 indicates that the user has restarted the browser, and if the cookie does not exist, it is assumed to be a new session. |
| CookieLawInfoConsent | 1 year | Records the default button state of the corresponding category & the status of CCPA. It works only in coordination with the primary cookie. |
| Cookie | Duration | Description |
|---|---|---|
| __cf_bm | 30 minutes | This cookie, set by Cloudflare, is used to support Cloudflare Bot Management. |
| __hssc | 30 minutes | HubSpot sets this cookie to keep track of sessions and to determine if HubSpot should increment the session number and timestamps in the __hstc cookie. |
| Cookie | Duration | Description |
|---|---|---|
| __hstc | 5 months 27 days | This is the main cookie set by Hubspot, for tracking visitors. It contains the domain, initial timestamp (first visit), last timestamp (last visit), current timestamp (this visit), and session number (increments for each subsequent session). |
| _ga | 2 years | The _ga cookie, installed by Google Analytics, calculates visitor, session and campaign data and also keeps track of site usage for the site's analytics report. The cookie stores information anonymously and assigns a randomly generated number to recognize unique visitors. |
| _gid | 1 day | Installed by Google Analytics, _gid cookie stores information on how visitors use a website, while also creating an analytics report of the website's performance. Some of the data that are collected include the number of visitors, their source, and the pages they visit anonymously. |
| hubspotutk | 5 months 27 days | HubSpot sets this cookie to keep track of the visitors to the website. This cookie is passed to HubSpot on form submission and used when deduplicating contacts. |
